Improving Price Transparency around Generic Drugs for Payers
Payers stand to save billions on prescription drug costs with improved price transparency around generic drugs.

Source: Thinkstock
- Increasing pricing transparency around generic drugs could offer significant advantages to payers, including lower prices and more favorable reimbursement negotiations, according to a report from USC Brookings.
Better understanding of contracting surrounding generics, a process which is often hidden from a payer’s view, can shift the balance of negotiating power to the payer’s favor and lead to billions in reduced healthcare spending.
“A major cause of limitations in competitive generic drug pricing appears to be third-party payers’ lack of information about actual prices paid by retail pharmacies to wholesalers and manufacturers for generic ingredients,” the Brookings team said.
“To the extent that providing health plans with additional information leads to lower reimbursement to retail and mail-order pharmacies, health spending would decline, with savings of $4 billion for every $1 reduction in the average reimbursement for a generic prescription,” the team added.
Payers need to be aware of the total reimbursement a pharmacy receives from both a health plan and a patient when filling a prescription, as well as how much a patient pays for prescription cost-sharing
Payers should also be aware of a generic drug’s ingredient costs as well as the profit a manufacturer receives for producing and selling a generic drug.
Changes in policy and legislation along with increased federal oversight could also play a large role in improving generic price transparency for payers, the report suggested.
Payers stand to benefit from a generic drug price index to determine how wholesale pricing, average manufacturer price, and related measures help establish national retail prices.
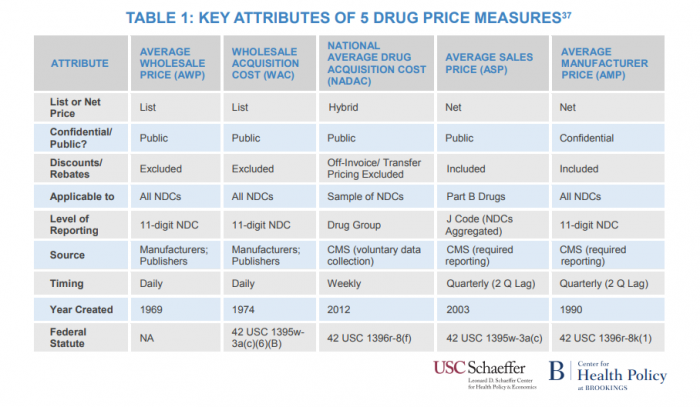
Source: USC Brookings
The USC Brookings team suggests that national generic licensing conditions should require each wholesaler to use a national drug code (NDC) to electronically report price concessions to CMS on a bi-weekly basis. CMS could also collect, store, and analyze NDC data under these licensing conditions.
The team also proposed issuing rules and guidance on how to define generic drug estimates and protect data, so that manufacturer data could not be used by competitors to reverse-engineer products.
CMS could also create special rules for concentrated market segments to preclude limiting competition, such as might occur for a particular medicine with only one manufacturer. The organization could also calculate reportable production costs through proposed rules and NDC data collection to calculate expenses of manufacturing ingredients, dosages, and other applicable market factors.
With all this data at their disposal, CMS could disclose the average costs to produce and sell generic drugs at retail pharmacies and report the data on a biweekly basis to health plans.
CMS could then charge participating plans a user fee to cover ongoing operating costs. Even with administrative fees, payers can expect to save billions on prescription drug spending, which already accounts for 10 percent of national spending.
“Policymakers should take steps to increase competition by making actual generic drug pricing information selectively available to third-party payers through a thoughtful and well-designed program that will allay antitrust concerns that greater transparency could diminish rather than enhance competition,” the report concluded.
